
Project
Medical device for reconstruction of spinal cord
Project Summary
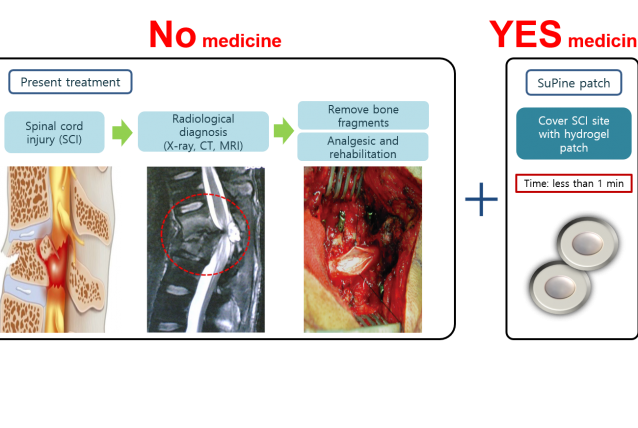
⦁ Approximately 500,000 new spinal cord injury occurs worldwide. However, spinal cord injury is incurable yet. Thus, we developed a hydrogel patch (SuPine patch) that can form a favorable environment for regeneration of injured spinal cord neurons. SuPine patch is ready-to-use at emergency and does not cause any damage while implanting it on the spinal cord.
⦁ SuPine Patch is made up of FDA approved ingredients. With the FDA Humanitarian Device Exemption program, SuPine Patch may directly enter clinical trail phase III once it enters clinical trials.
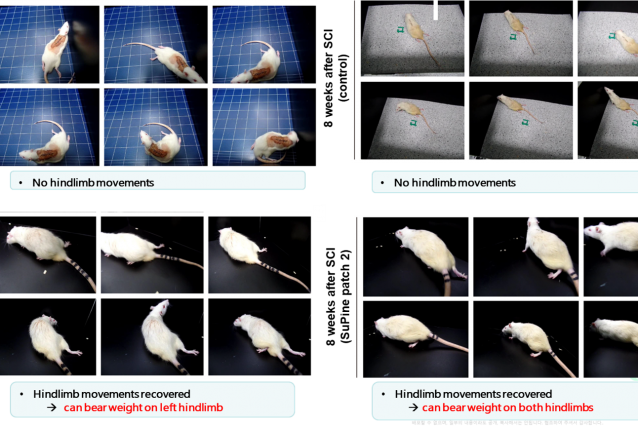
⦁ SuPine Patch shows explicit motor recovery in pre-clinical trials.
⦁ SuPine Patch is made up of FDA approved ingredients. With the FDA Humanitarian Device Exemption program, SuPine Patch may directly enter clinical trail phase III once it enters clinical trials.
⦁ SuPine Patch shows explicit motor recovery in pre-clinical trials.
Business Model
Revenue Model:
Licensing out to domestic and oversea pharmaceutical companies for FDA approval and market entrance.
Target Customer:
Pharmaceutical companies, neurosurgeon, Emergency
Distribution Channels:
Pharmaceutical companies, Hospitals, Army hospital
Licensing out to domestic and oversea pharmaceutical companies for FDA approval and market entrance.
Target Customer:
Pharmaceutical companies, neurosurgeon, Emergency
Distribution Channels:
Pharmaceutical companies, Hospitals, Army hospital
Main Achievements
Follow-up Investment:
Tapping VC and pharmaceutical companies for follow-up investment
Employment:
1
Dissertations or IP:
1 IP applied, PCT, 1 Journal
Other Achievements:
A Minister of Ministry of SMEs and Startups award,
Winner of 2017 U-Star ( $20,000 prize)
Tapping VC and pharmaceutical companies for follow-up investment
Employment:
1
Dissertations or IP:
1 IP applied, PCT, 1 Journal
Other Achievements:
A Minister of Ministry of SMEs and Startups award,
Winner of 2017 U-Star ( $20,000 prize)
Date of Establishment2017-11-21
AcceleratorSunbo angel partners
StageBiomedical
Date of TIPS Selection2017-11-30
